
Published On: 05 Jun, 2025 4:02 PM
|
Updated On: 13 Jun, 2025 11:15 AM
FAERS Database Insights: Revisiting the β-Blocker and Asthma Association
Mario Cazzola a*, Josuel Ora b, Luigino Calzetta c, Paola Rogliani a,b, Maria Gabriella Matera d
a Unit of Respiratory Medicine, Department of Experimental Medicine, University of Rome ‘Tor Vergata’, Rome, Italy
b Division of Respiratory Medicine, University Hospital Policlinico ‘Tor Vergata’, Rome, Italy
c Department of Medicine and Surgery, Respiratory Disease and Lung Function Unit, University of Parma, Parma, Italy
d.Unit of Pharmacology, Department of Experimental Medicine, University of Campania ‘Luigi Vanvitelli’, Naples, Italy
Abstract
Introduction: β-Blockers play a vital role in managing cardiovascular conditions but may trigger respiratory complications, particularly when non-selective β-blockers are used. Their safety profile in asthmatic patients remains a topic of ongoing debate.
Objective: This study aimed to explore the association between various classes of β-blockers and the occurrence of asthma and asthma-like adverse events (AEs), utilizing data from the Food and Drug Administration's Adverse Event Reporting System (FAERS).
Methods: β-Blockers were classified based on the European Society of Cardiology guidelines and the Vashistha and Kumar classification. Disproportionality analysis was employed to assess risk across different β-blocker classes, using the reporting odds ratio (ROR) as the key measure.
Results: Out of 251,145 β-blocker-related AEs reported, 4,104 were linked to asthma. Selective β1-blockers showed a higher asthma risk signal (ROR: 1.15) compared to non-selective β-blockers (ROR: 0.90). The lowest risk was observed with α- and β-blockers (ROR: 0.51). Further analysis using the Vashistha and Kumar classification revealed varying risk profiles even among drugs within the same class. Dual α- and β-blockers, as well as hydrophilic and lipophilic β-blockers, demonstrated lower asthma risks, while selective β1-blockers posed higher risks regardless of their intrinsic sympathomimetic activity.
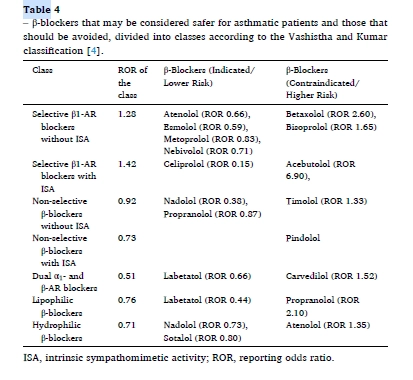
Conclusion: While the risks identified through disproportionality analysis are considered potential rather than confirmed, the study underscores the importance of careful β-blocker selection for asthmatic patients or those susceptible to asthma. Despite limitations in FAERS data, the findings highlight significant variations in risk among β-blocker classes, providing valuable insights for clinical decision-making. Safer options for asthmatic patients include esmolol, metoprolol, nebivolol, and nadolol, while betaxolol, bisoprolol, timolol, and propranolol should be used with caution or avoided.
Introduction
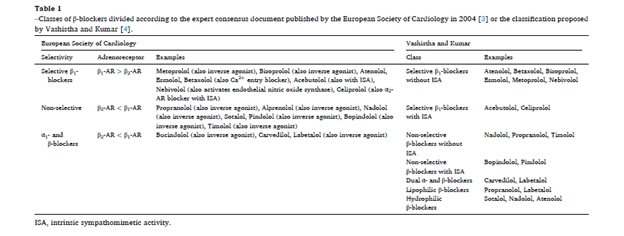
β-Blockers are a widely prescribed class of medications for managing cardiovascular diseases (CVDs), including hypertension, angina, and heart failure [1]. They function by blocking β-adrenoceptors (β-ARs), which are crucial for regulating cardiovascular responses through the sympathetic nervous system [2]. According to the European Society of Cardiology (ESC) classification, β-blockers are categorized into three groups: non-selective (β1 + β2)-blockers, selective β1-blockers, and α1- and β-blockers [3] (Table 1).
Alternatively, Vashistha and Kumar’s classification offers a more comprehensive approach, dividing β-blockers into seven classes based on pharmacokinetics, pharmacodynamics, and mechanisms of action: selective β1-blockers with and without intrinsic sympathomimetic activity (ISA), non-selective β-blockers with and without ISA, dual α- and β-blockers, lipophilic β-blockers, and hydrophilic β-blockers [4] (Table 1).
While β-blockers remain highly effective for CVD management, they have been associated with respiratory adverse events (AEs), such as bronchospasm and worsening respiratory symptoms. Additionally, they can diminish the effectiveness of β2-agonists, which are commonly used to manage asthma and chronic obstructive pulmonary disease (COPD) [1,2]. In some cases, β-blockers may trigger moderate to severe asthma exacerbations and increase mortality risk among patients with advanced COPD [1,5]
Cardioselective β1-blockers are generally safe and effective for patients with cardiovascular diseases (CVDs), even in those with respiratory conditions like COPD and asthma. While β-blockers can occasionally impair pulmonary function, they are not contraindicated in these conditions. The European Society of Cardiology (ESC) guidelines recommend initiating cardioselective β1-blockers at low doses with careful monitoring for airway obstruction symptoms, such as wheezing and shortness of breath [6].
Studies indicate that β-blockers, including both β1-selective and nonselective agents, not only reduce all-cause and in-hospital mortality in COPD patients but also offer cardiovascular benefits without significant respiratory compromise [7]. However, nonselective β-blockers or those with low β1-selectivity can exacerbate asthma symptoms in patients with classical pulmonary asthma [7]. The ESC guidelines further highlight that low-dose cardioselective β1-blockers may be cautiously used in patients with chronic asthma [8].
Although both cardioselective and non-cardioselective β-blockers can lead to slight reductions in FEV1 and FVC values, only non-cardioselective β-blockers significantly lower the FEV1/FVC ratio, indicating notable airflow obstruction [9]. Importantly, any initial decline in lung function with β-blockers tends to be temporary, even in patients with reactive airway disorders [10].
To further evaluate respiratory risks, the World Health Organization’s VigiBase database was analyzed for reports of fatal asthma or bronchospasm associated with cardioselective β1-blockers. Out of 583 asthma cases and 1,015 bronchospasm cases, only 6 and 12 deaths were reported, respectively, often involving pre-existing lung disease and concomitant conditions like sepsis, pneumonia, or cancer [11]. The data suggested that bronchospasm was rarely the primary cause of death, and the β-blockers themselves were not directly implicated [12,13].
While asthma is not an absolute contraindication for cardioselective β1-blockers, careful prescribing and patient monitoring remain essential [7,8]. The choice of β1-blocker should prioritize safety, considering the minimal but present risk of bronchoconstriction in asthmatic patients [15]. To further refine this approach, the study assessed data from the FDA's Adverse Event Reporting System (FAERS) to identify β-blockers with the least risk of asthma-related adverse events.
2. Methods:
2.1. Overview of the FAERS Database
The FDA Adverse Event Reporting System (FAERS) is a publicly accessible repository of adverse event (AE) reports submitted by healthcare providers, consumers, and manufacturers via the MedWATCH program [16]. It provides detailed information on drug and biologic product safety, including AE types, patient demographics, and relevant clinical data. FAERS plays a crucial role in post-marketing surveillance, offering insights into medication safety profiles through real-world exposure and a large, diverse sample size [17].
2.2. Data Collection and Categorization
Adverse events were categorized using the preferred term (PT) level of the Medical Dictionary for Regulatory Activities (MedDRA), version 26.1. The analysis focused on PTs under the “respiratory, thoracic, and mediastinal disorders” category, including terms such as “allergic bronchitis,” “asthma,” “bronchial hyperreactivity,” “bronchospasm,” “wheezing,” and related conditions. Each report could include one or more respiratory AEs, as emphasized by FAERS.
β-blockers were classified using both the European Society of Cardiology (ESC) and the Vashistha and Kumar classifications [3,4]. While the ESC classification provides broader categories suitable for general understanding, the Vashistha and Kumar classification offers more detailed risk profiles, enhancing clinical decision-making accuracy.
2.3. Signal Identification and Analysis
Disproportionality analysis was employed to identify potential drug-related AE signals [18,19]. This method compares the incidence of specific AEs associated with a particular drug to the incidence across all other drugs. A signal is considered statistically significant when the observed incidence exceeds the background rate, meeting predefined thresholds.
Two primary metrics recommended by the European Medicines Agency were utilized:
- Reporting Odds Ratio (ROR): The ratio of reported cases for a given drug compared to all other drugs. A lower bound of the 95% confidence interval (CI) greater than 1, with at least three cases, indicates statistical significance [20–22].
- Proportional Reporting Ratio (PRR): The ratio of the proportion of a specific AE in individuals exposed to a drug compared to those exposed to other drugs [23].
Although both measures are widely accepted, the ROR is often preferred due to its reduced bias and superior signal detection, as demonstrated by Rothman et al. and Chen et al. [24,25]. Additionally, ROR facilitates multivariate logistic regression, allowing for the evaluation of confounding and interaction effects [21].
Given the inability to estimate the at-risk population in this study, odds ratios were used exclusively for disproportionality analysis, assessing the association between β-blockers and asthma-related AEs [26].
2.4. Ethical Statement
The FAERS database contains anonymized data; therefore, ethics committee approval was not required for this analysis.
Results
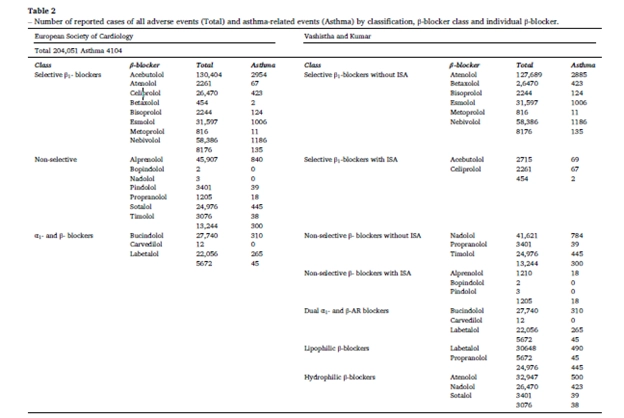
A total of 251,145 adverse events (AEs) related to β-blockers were reported to FAERS by March 31, 2024. Among these, 4,104 cases were identified as asthma-related events. The analysis categorized β-blockers based on two major classifications: the European Society of Cardiology (ESC) classification and the Vashistha and Kumar classification to assess the asthma-related risks associated with each category.(Table 2)
3.1. β-Blockers Categorized According to ESC Classification
The ESC classification divides β-blockers into three major categories:
1. Selective β1-blockers
2. Non-selective β-blockers
3. Dual α- and β-blockers
Key Findings:
- Selective β1-blockers showed a higher risk of asthma-related events than expected.
- The reporting odds ratio (ROR) was 1.15 (95% CI: 1.08–1.23), indicating that patients using selective β1-blockers were 15% more likely to report asthma-related AEs compared to non-users.
- Non-selective β-blockers, on the other hand, exhibited a lower risk of asthma-related events.
- The ROR was 0.90 (95% CI: 0.83–0.97), suggesting a 10% lower likelihood of reporting asthma-related AEs compared to non-users.
- The 95% CI being below 1 confirms the statistical significance, making it an unexpected yet important finding that non-selective β-blockers may be safer for asthma patients than selective β1-blockers.
- Dual α- and β-blockers demonstrated a significant reduction in asthma-related AEs.
- The ROR was 0.51 (95% CI: 0.46–0.58), meaning patients using these drugs had a 49% lower risk of reporting asthma-related events compared to non-users.
- The robust confidence interval supports the credibility of this observation.
Disproportionality rate (ROR with 95 % CI) of asthma and asthma-like AEs registered in the FAERS database up to March 31, 2024 by β-blocker class according to the classification of the European Society
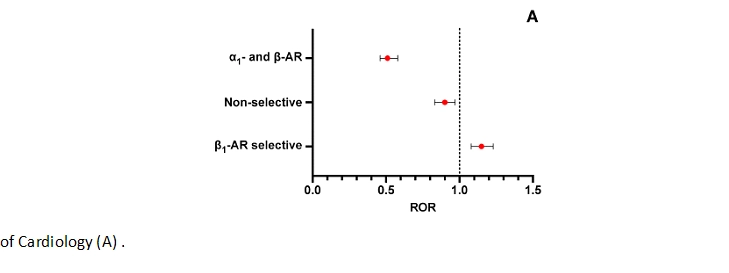
3.2. β-Blockers Categorized According to the Vashistha and Kumar Classification
This classification allowed for a more granular analysis, assessing asthma risk based on specific β-blocker properties, including hydrophilicity, lipophilicity, intrinsic sympathomimetic activity (ISA), and selectivity.
Disproportionality rate (ROR with 95 % CI) of asthma and asthma-like AEs registered in the FAERS database up to March 31, 2024 by β-blocker class according to proposed by Vashistha and Kumar (B) compared with all other reports of AEs
Low-Risk Groups:
1. Dual α- and β-blockers
- ROR 0.51 (95% CI: 0.46–0.58) → Highly significant reduction in asthma risk.
2. Hydrophilic β-blockers
- ROR 0.71 (95% CI: 0.65–0.79) → Moderately reduced risk of asthma-related events.
3. Lipophilic β-blockers
- ROR 0.76 (95% CI: 0.69–0.84) → Slightly reduced risk, but still statistically significant.
Variable-Risk Groups:
- Non-selective β-blockers without ISA showed a neutral to slightly protective effect.
- ROR 0.92 (95% CI: 0.85–1.00)
- However, individual drugs within this class showed varying risk profiles:
- Timolol: Increased risk (ROR 1.33, 95% CI: 1.15–1.55).
- Propranolol: Neutral to slightly increased risk (ROR 0.87, 95% CI: 0.76–1.01).
- Nadolol: Low risk (ROR 0.38, 95% CI: 0.28–0.53).
Non-selective β-blockers with ISA showed a trend towards a reduced risk, but statistical significance was not achieved due to a wide confidence interval (ROR 0.73, 95% CI: 0.46–1.17).
- Only pindolol had asthma-related reports in this category.
- High-Risk Groups:1. Selective β1-blockers with ISA
- ROR 1.42 (95% CI: 1.33–1.52) → 42% higher risk of asthma-related AEs.
- This is statistically significant, confirming an increased asthma risk.
2. Selective β1-blockers without ISA- ROR 1.28 (95% CI: 1.00–1.62) → Increased risk, though the upper limit of the confidence interval suggests borderline significance.
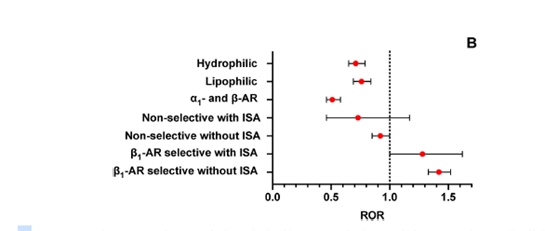
- Individual drug differences within this class:
- Low-risk drugs:
- Atenolol: ROR 0.66 (95% CI: 0.59–0.72)
- Esmolol: ROR 0.59 (95% CI: 0.32–1.07)
- Metoprolol: ROR 0.83 (95% CI: 0.77–0.89)
- Nebivolol: ROR 0.71 (95% CI: 0.60–0.85)
- High-risk drugs:
- Betaxolol: ROR 2.60 (95% CI: 2.16–3.13)
- Bisoprolol: ROR 1.65 (95% CI: 1.53–1.78)
3. Selective β1-blockers with ISA (Individual Drugs)- Acebutolol: Significantly higher risk (ROR 6.90, 95% CI: 1.68–28.27).
- Celiprolol: Very low risk (ROR 0.15, 95% CI: 0.04–0.59).
Disproportionality rate (ROR with 95 % CIs) of reports of asthma and asthma-like AEs recorded in the FAERS database up to March 31, 2024 for each β-blocker in the context of its class according to the Vashistha and Kumar classification, compared with all other reports of AEs associated with β-blocker use.
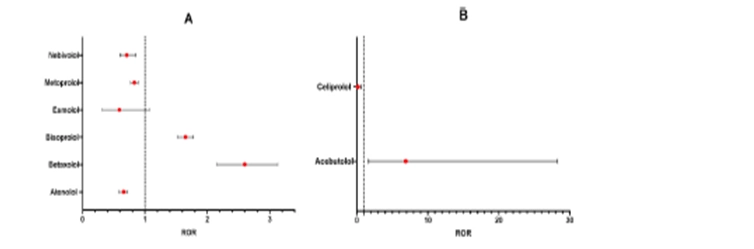
A. Selective β1-blockers without ISA; B, Selective β1-blockers with ISA;

C, Non-selective β-blockers without ISA; D, dual α1-and β-blockers; E, lipophilic β-blockers; F, hydrophilic β-blockers.
Additional Drug-Specific Findings:
- Among dual α- and β-blockers:
- Labetalol (ROR 0.66, 95% CI: 0.48–0.90) had a lower risk.
- Carvedilol (ROR 1.52, 95% CI: 1.11–2.09) showed an increased risk, contrary to expectations.
- Among hydrophilic β-blockers:
- Atenolol (ROR 1.35, 95% CI: 1.06–1.72) showed an increased risk.
- Sotalol (ROR 0.80, 95% CI: 0.57–1.11) and Nadolol (ROR 0.73, 95% CI: 0.53–1.02) showed lower risks.
- Among lipophilic β-blockers:
- Propranolol (ROR 2.10, 95% CI: 1.55–2.87) showed a notably high risk of asthma-related AEs.
- However, when considered in the non-selective β-blockers without ISA category, propranolol had a neutral to slightly increased risk (ROR 0.87, 95% CI: 0.76–1.01).
Discussion
The use of β-blockers, a cornerstone in the management of several CVDs including hypertension, angina, heart failure, and arrhythmias, requires careful consideration in patients with asthma or other reactive airway diseases due to the potential risk of bronchoconstriction and exacerbation of respiratory symptoms. As the safety margin of these drugs is narrower than in COPD, the latest European Society of Hypertension guidelines recommend against using β-blockers to treat hypertension in patients with asthma [27].
Our analysis suggests that selective β1-blockers without ISA should be avoided in asthma patients due to an appreciably elevated risk, whereas selective β1-blockers with ISA should be used with caution due to a moderately elevated risk. However, this finding contrasts with the evidence from a meta-analysis of patients treated with long-term cardioselective β1-blockers, which indicated no increased risk of exacerbation, death, or asthma [28]. Analysis of pharmacovigilance databases was also very reassuring regarding the tolerability of selective β1-blockers in asthmatics [28].
Moreover, findings from the 2014 systematic review and meta-analysis by Morales et al. further support this conclusion. Their study highlighted that while selective β-blockers are generally better tolerated, they are not completely risk-free. The risk from acute exposure can be minimized by using the smallest effective dose and β-blockers with greater β1-selectivity. Importantly, β-blocker-induced bronchospasm responded partially to β2-agonists, with nonselective β-blockers blunting the response more than selective ones. This underscores the need for individualized risk assessment when prescribing β-blockers to asthma patients. [59].
Additionally, Bennett et al. conducted a comprehensive literature review and global pharmacovigilance search, revealing no published reports of severe or fatal asthma exacerbations associated with cardioselective β1-blockers. Supporting this, three large observational studies found no increase in asthma exacerbations among patients treated with these β1-blockers, reinforcing their potential safety profile in asthma management. [60].
Similarly, the meta-analysis by Huang et al. identified increased asthma risk with oral timolol and intravenous propranolol compared to placebo, while β1-selective agents like celiprolol, bisoprolol, and atenolol were associated with a lower incidence of asthma attacks. These findings emphasize the importance of selecting appropriate β-blockers for asthma patients to minimize respiratory risks[61].
Nevertheless, one of the more intriguing aspects of our analysis was the emergence of a number of differences between the individual drugs in the two classes. To explain these differences, it could be argued that the pharmacology of cardioselective β1-blockers suggests that none of the currently available β-blockers exhibit absolute selectivity for β1-ARs [2]. While cardioselective β1-blockers tend to demonstrate less pronounced β2-AR antagonism compared to non-selective β-blockers, several of them show minimal preference for β1-ARs over β2-ARs [29].
The aforementioned hypothesis does not, however, account for the AEs observed with bisoprolol and acebutolol, which have been demonstrated to possess high affinity for β1-ARs [2]. It is possible that additional pharmacological properties are involved [30]. Nebivolol is a highly selective β1-AR blocker that also activates endothelial nitric oxide synthase. The potential role of nitric oxide in airway control is well established [31]. Celiprolol is a β1-AR blocker with partial β2-agonist activity, weak blockade of postjunctional α1-ARs, and prejunctional α2-AR effects.
Non-selective β-AR blockers without ISA also demonstrated considerable variability. They are typically avoided in asthma patients due to the inherent risk of β2-AR blockade in the airways, which may result in bronchoconstriction [2]. However, while timolol exhibited a markedly elevated risk of asthma-related events, nadolol showed a significantly reduced risk. Timolol is the most prescribed non-selective β-AR blocker eye drop [32]. It has greater selectivity for the β2-AR than other commonly used non-selective β-AR blockers [32]. Nadolol is also an inverse agonist [33]. This implies that when it binds to β-ARs, it not only blocks the effects of adrenaline and noradrenaline, but also induces a reduction in basal receptor activity below the level observed in the absence of any ligand (agonist or antagonist), thereby shifting the balance of spontaneously active receptors towards the inactive state [34]. By attenuating basal receptor activity, inverse agonists may potentially elicit bronchodilation in the absence of external agonists [35]. There is evidence that nadolol has beneficial effects on airway hyperresponsiveness [36].
Although there is evidence that the infusion of propranolol is associated with a significantly higher risk of developing an asthma attack, predominantly in patients with a pre-existing history of asthma [37], our data indicate that this β-blocker, when considered within the broader category of non-selective β-AR blockers without ISA, exhibits a neutral to slightly increased risk of asthma-related events. It is noteworthy that the administration of propranolol in patients with hypertension and anxiety was associated with increased β2-AR bronchodilating function, which is contrary to the anticipated outcome [38]. It can be reasonably deduced that propranolol may not fully block the activity of β2-ARs, as it has the potential to induce inverse agonism [2]. In a rat model of passive cigarette smoking, Guo and colleagues observed that one-month course of propranolol treatment enhanced β2-AR-mediated relaxation [39]. Concurrently, it attenuated the contractile response to acetylcholine by reducing noradrenaline. In contrast, metoprolol, which is a selective β1-AR blocker with ISA, reduced blood catecholamine levels but did not increase airway smooth muscle responsiveness to β2-AR agonists. A single-center, randomized, double-blind, placebo-controlled trial conducted in the United Kingdom revealed that patients with chronic asthma who received 80 mg/d of propranolol did not experience any AE [40,41]. However, propranolol is associated with a higher risk of asthma-related problems if it is classified as a lipophilic β-blocker. Being lipophilic, propranolol is capable of crossing cell membranes and affecting lung tissue, which may result in a worsening of asthma symptoms. Conversely, β-blockers with a hydrophilic profile, such as sotalol and nadolol, demonstrated a lower risk, potentially due to their limited penetration into lung tissue, which in turn leads to a diminished likelihood of bronchoconstriction.
There was little data on non-selective β-AR blockers with ISA in the FAERS database, but these drugs should generally be avoided in asthma patients as they can worsen respiratory symptoms.
The dual α- and β-AR blockers demonstrated a significantly lower risk of AEs, which may be attributed to their ability to cause vasodilation and reduce airway resistance. However, carvedilol exhibited a higher risk, whereas labetalol demonstrated a comparatively lower risk. The differential risk profiles of carvedilol and labetalol for asthma-related AEs can be ascribed to their distinct pharmacological properties. The higher risk of carvedilol can be attributed to its significant β2-AR antagonism [25], which can lead to bronchoconstriction. Conversely, the lower risk of labetalol may be due to its balanced adrenergic blockade. It exhibits a higher affinity for α1-ARs compared to β1- or β2-ARs and a lower affinity for β2-ARs than carvedilol [29,42]. This difference should be carefully considered when selecting a dual α- and β-AR blocker for patients with asthma or those at risk of respiratory complications.
In any case, when interpreting the results of our analysis, it is important to recognize the limitations associated with the FAERS data. Thus, although the results of this study are compelling, they do not provide conclusive evidence that β-blockers lead to asthma-related AEs.
The FDA itself has highlighted that relying on FAERS data alone is an inadequate approach for determining the safety profile of a drug [16,43]. Indeed, there is currently no definitive evidence linking the reported event, whether an AE or a medication error, to the drug in question. This is because the FDA does not require definitive evidence establishing a causal link between a product and an event. AE reports are voluntarily submitted by healthcare providers, consumers, and manufacturers, which may result in false, exaggerated, inaccurate, incomplete, or delayed information. The information provided in these reports is limited to the observations and perspectives of the reporters. Although it is recommended that both consumers and healthcare professionals report any AEs, it is essential to recognize that such reactions may be linked to the underlying disease being addressed, may result from other medications taken simultaneously, or may occur due to various other reasons. In any case, reports are often not sufficiently detailed for accurate evaluation, nor are they subject to medical review, which increases the risk of misclassification.
FAERS data cannot establish a definitive causal link between a drug and an adverse event (AE) due to limited patient population data, underreporting, duplicate reports, and inherent biases in spontaneous reporting. Reporting is influenced by factors like time on the market and media coverage, known as the Weber effect and notoriety effect, respectively. AE signals may rise after a drug’s launch and fluctuate over time, while widespread reporting can mask signals, known as the cloaking effect. Despite these limitations, the FAERS analysis highlights variability in asthma-related AE risk across β-blocker classes, emphasizing the need for careful β-blocker selection, especially in patients with respiratory conditions.(44-50) Table 3
Conclusion
The Global Initiative for Asthma underscores the potential risks of β-blockers in asthma patients, including bronchospasm and, in rare cases, asthma-related mortality. Despite these concerns, β-blockers offer significant cardiovascular benefits, such as reducing mortality in asthmatics hospitalized for acute coronary events. This duality has led to inconsistent guidelines: Japanese recommendations advocate cautious use of β1-selective blockers in patients with comorbid cardiovascular disease (CVD), while the British Thoracic Society and NHLBI advise complete avoidance, including ophthalmic formulations.
These conflicting guidelines often result in the underuse of β-blockers, depriving patients with both CVD and respiratory conditions of potentially life-saving treatment. Given that up to one-third of patients with heart failure and reduced ejection fraction also have respiratory comorbidities, asthma or COPD should not automatically contraindicate β-blocker use. Instead, prescribing should be guided by thorough clinical evaluation, including spirometry, bronchodilator reversibility testing, and assessment of airway hyperresponsiveness.
Our real-world data highlight that esmolol, metoprolol, nebivolol, and nadolol are associated with a lower risk of asthma-related adverse events and should be preferred in patients with respiratory conditions. In contrast, betaxolol, bisoprolol, acebutolol, propranolol, and timolol carry a higher risk and should be avoided. Atenolol and bisoprolol warrant cautious use, particularly in patients with a history of reactive airway disease. (Table 4)
Recent network meta-analyses further suggest that while oral timolol and intravenous propranolol increase asthma risk, celiprolol, bisoprolol, practolol, and sotalol may reduce it. However, the identification of bisoprolol as the third most prevalent β1-blocker associated with asthma episodes in the VigiBase analysis warrants further investigation, given conflicting evidence regarding its safety in mild-to-moderate asthma.
In conclusion, β-blocker use in asthma patients should be individualized, considering the specific drug’s safety profile, patient characteristics, and asthma severity. Our findings, combined with existing evidence, provide critical insights to inform clinical decision-making in this complex therapeutic landscape.
References
1. J. Ora, F. Cavalli, M. Cazzola, Management of patients with asthma or COPD and cardiovascular disease: risks versus benefits, in: M.´A. Martínez-García, J.-L. P´epin, M. Cazzola (Eds.), Cardiovascular Complications of Respiratory Disorders (ERS Monograph), European Respiratory Society, Sheffield, 2020, pp. 66–81, https:// doi.org/10.1183/2312508X.10027419.
2. M.G. Matera, P. Rogliani, L. Calzetta, M. Cazzola, An overview of the efficacy and safety of β2-adrenoceptor antagonists for the treatment of chronic obstructive pulmonary disease, Expet Opin. Drug Saf. 23 (7) (2024) 833–844, https://doi.org/ 10.1080/14740338.2024.2362817.
3. J. L´opez-Send´on, K. Swedberg, J. McMurray, et al., Expert consensus document on beta-adrenergic receptor blockers, Eur. Heart J. 25 (15) (2004) 1341–1362, https://doi.org/10.1016/j.ehj.2004.06.002.
4. V.K. Vashistha, A. Kumar, Stereochemical facets of clinical β-blockers: an overview, Chirality 32 (5) (2020) 722–735, https://doi.org/10.1002/chir.23200.
5. M. Cazzola, L. Calzetta, B. Rinaldi, et al., Management of chronic obstructive pulmonary disease in patients with cardiovascular diseases, Drugs 77 (7) (2017) 721–732, https://doi.org/10.1007/s40265-017-0731-3.
6. T.A. McDonagh, M. Metra, M. Adamo, et al., 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC, Eur. J. Heart Fail. 24 (1) (2022) 4–131, https://doi. org/10.1002/ejhf.2333.
7. G. Mancia, S.E. Kjeldsen, R. Kreutz, A. Pathak, G. Grassi, M. Esler, Individualized beta-blocker treatment for high blood pressure dictated by medical comorbidities: indications beyond the 2018 European society of Cardiology/European society of hypertension guidelines, Hypertension 79 (6) (2022) 1153–1166, https://doi.org/ 10.1161/HYPERTENSIONAHA.122.19020.
8. J.W. McEvoy, C.P. McCarthy, R.M. Bruno, et al., 2024 ESC Guidelines for the management of elevated blood pressure and hypertension, Eur. Heart J. 45 (38) (2024) 3912–4018, https://doi.org/10.1093/eurheartj/ehae178.
9. D.W. Loth, G.G. Brusselle, L. Lahousse, A. Hofman, H.G. Leufkens, B.H. Stricker, β-Adrenoceptor blockers and pulmonary function in the general population: the Rotterdam Study, Br. J. Clin. Pharmacol. 77 (1) (2014) 190–200, https://doi.org/ 10.1111/bcp.12181.
10. T.M. Parekh, E.S. Helgeson, J. Connett, et al., Lung function and the risk of exacerbation in the β-blockers for the prevention of acute exacerbations of chronic obstructive pulmonary disease trial, Ann. Am. Thorac. Soc. 19 (10) (2022) 1642–1649, https://doi.org/10.1513/AnnalsATS.202109-1042OC.
11. M. Bennett, C.L. Chang, M. Tatley, R. Savage, R.J. Hancox, The safety of cardioselective β1-blockers in asthma: literature review and search of global pharmacovigilance safety reports, ERJ Open Res. 7 (1) (2021) 801–2020, https:// doi.org/10.1183/23120541.00801-2020.
12. Uppsala Monitoring Centre, About VigiBase, Available at: https://who-umc.org/ vigibase/. (Accessed 22 October 2024).
13. A. Tiotiu, P. Novakova, K. Kowal, et al., Beta-blockers in asthma: myth and reality, Expet Rev. Respir. Med. 13 (9) (2019) 815–822, https://doi.org/10.1080/ 17476348.2019.1649147.
14. M. Cazzola, N.A. Hanania, P. Rogliani, M.G. Matera, Cardiovascular disease in asthma patients: from mechanisms to therapeutic implications, Kardiol. Pol. 81 (3) (2023) 232–241, https://doi.org/10.33963/KP.a2023.0038.
15. M. Cazzola, P. Noschese, M. D’Amato, et al., Comparison of the effects of single oral doses of nebivolol and celiprolol on airways in patients with mild asthma, Chest 118 (5) (2000) 1322–1326, https://doi.org/10.1378/chest.118.5.1322.
16. U.S. Food and Drug Administration, FDA adverse event reporting system (FAERS) public dashboard, Available at: https://www.fda.gov/drugs/questions-and-ans wers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-syste m-faers-public-dashboard, , 2023. (Accessed 6 July 2024).
17. S.D. Silberstein, S. Reshef, J.M. Cohen, et al., Adverse events reported with therapies targeting the CGRP pathway during the first 6 months post-launch: a retrospective analysis using the FDA Adverse Events Reporting System, Adv. Ther. 40 (2) (2023) 445–459, https://doi.org/10.1007/s12325-022-02346-4.
18. C. Michel, E. Scosyrev, M. Petrin, R. Schmouder, Can disproportionality analysis of post-marketing case reports be used for comparison of drug safety profiles? Clin. Drug Invest. 37 (5) (2017) 415–422, https://doi.org/10.1007/s40261-017-0503-6.
19. O. Caster, Y. Aoki, L.M. Gattepaille, B. Grundmark, Disproportionality analysis for pharmacovigilance signal detection in small databases or subsets: recommendations for limiting false-positive associations, Drug Saf. 43 (5) (2020) 479–487, https://doi.org/10.1007/s40264-020-00911-w.
20. European Medicines Agency, Screening for adverse reactions in EudraVigilance. EMA/849944/2016, 3-33. 2024. Available at: https://www.ema.europa.eu/en/ documents/other/screening-adverse-reactions-eudravigilance_en.pdf, , 2016. (Accessed 6 July 2024).
21. E.P. van Puijenbroek, A. Bate, H.G. Leufkens, M. Lindquist, R. Orre, A.C. Egberts, A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions, Pharmacoepidemiol. Drug Saf. 11 (1) (2002) 3–10, https://doi.org/10.1002/pds.668.
22. Y. Zhai, X. Ye, F. Hu, et al., Endocrine toxicity of immune checkpoint inhibitors: a real-world study leveraging US Food and Drug Administration adverse events reporting system, J. Immunother. Cancer 7 (1) (2019) 286, https://doi.org/ 10.1186/s40425-019-0754-2.
23. S.J. Evans, P.C. Waller, S. Davis, Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports, Pharmacoepidemiol. Drug Saf. 10 (6) (2001) 483–486, https://doi.org/10.1002/ pds.677.
24. K.J. Rothman, S. Lanes, S.T. Sacks, The reporting odds ratio and its advantages over the proportional reporting ratio, Pharmacoepidemiol. Drug Saf. 13 (8) (2004) 519–523, https://doi.org/10.1002/pds.1001.
25. Y. Chen, J.J. Guo, M. Steinbuch, X. Lin, C.R. Buncher, N.C. Patel, Comparison of sensitivity and timing of early signal detection of four frequently used signal detection methods, Pharmaceut. Med. 22 (2008) 359–365, https://doi.org/ 10.1007/BF03256733.
26. P. Sedgwick, Case-control studies: measures of risk, BMJ 346 (2013) f1185, https://doi.org/10.1136/bmj.f1185.
27. G. Mancia, R. Kreutz, M. Brunstr¨om, et al., 2023 ESH guidelines for the management of arterial hypertension the task force for the management of arterial hypertension of the European society of hypertension: endorsed by the international society of hypertension (ISH) and the European renal association (ERA), J. Hypertens. 41 (12) (2023) 1874–2071, https://doi.org/10.1097/ HJH.0000000000003480.
28. M. Bennett, C.L. Chang, M. Tatley, R. Savage, R.J. Hancox, The safety of cardioselective β1-blockers in asthma: literature review and search of global pharmacovigilance safety reports, ERJ Open Res. 7 (1) (2021) 801–2020, https:// doi.org/10.1183/23120541.00801-2020.
29. J.G. Baker, The selectivity of β-adrenoceptor antagonists at the human beta β1, β2 and β3 adrenoceptors, Br. J. Pharmacol. 144 (3) (2005) 317–322, https://doi.org/ 10.1038/sj.bjp.0706048.
30. M. Cazzola, P. Noschese, M. D’Amato, G. D’Amato, Comparison of the effects of asthma, Chest 118 (5) (2000) 1322–1326, https://doi.org/10.1378/ chest.118.5.1322.
31. M.G. Matera, Nitric oxide and airways, Pulm. Pharmacol. Ther. 11 (5–6) (1998) 341–348, https://doi.org/10.1006/pupt.1999.0173.
32. D.R. Morales, T. Dreischulte, B.J. Lipworth, P.T. Donnan, C. Jackson, B. Guthrie, Respiratory effect of beta-blocker eye drops in asthma: population-based study and meta-analysis of clinical trials, Br. J. Clin. Pharmacol. 82 (3) (2016) 814–822, https://doi.org/10.1111/bcp.13006.
33. H. Peng, R.A. Bond, B.J. Knoll, The effects of acute and chronic nadolol treatment on β2AR signaling in HEK293 cells, Naunyn-Schmiedeberg’s Arch. Pharmacol. 383 (2) (2011) 209–216, https://doi.org/10.1007/s00210-010-0591-9.
34. P. Chidiac, T.E. Hebert, M. Valiquette, M. Dennis, M. Bouvier, Inverse agonist activity of beta-adrenergic antagonists, Mol. Pharmacol. 45 (3) (1994) 490–499.
35. J.K. Walker, R.B. Penn, N.A. Hanania, B.F. Dickey, R.A. Bond, New perspectives regarding β2-adrenoceptor ligands in the treatment of asthma, Br. J. Pharmacol. 163 (1) (2011) 18–28, https://doi.org/10.1111/j.1476-5381.2010.01178.x.
36. N.A. Hanania, S. Singh, R. El-Wali, et al., The safety and effects of the beta-blocker, nadolol, in mild asthma: an open-label pilot study, Pulm. Pharmacol. Ther. 21 (1) (2008) 134–141, https://doi.org/10.1016/j.pupt.2007.07.002.
37. K.Y. Huang, P.T. Tseng, Y.C. Wu, et al., Do beta-adrenergic blocking agents increase asthma exacerbation? A network meta-analysis of randomized controlled trials, Sci. Rep. 11 (1) (2021) 452, https://doi.org/10.1038/s41598-020-79837-3.
38. D.R. Lima, P. Turner, Propranolol increases reduced beta-receptor function in severely anxious patients, Lancet 2 (8365–66) (1983) 1505, https://doi.org/10.1016/s0140-6736(83)90855-3
39. Y. Guo, Y. Zhang, N. Shen, et al., Effects of one month treatment with propranolol and metoprolol on the relaxant and contractile function of isolated trachea from rats exposed to cigarette smoke for four months, Inhal. Toxicol. 26 (5) (2014) 271–277, https://doi.org/10.3109/08958378.2014.885098.
40. W.J. Anderson, P.M. Short, P.A. Williamson, A. Manoharan, B.J. Lipworth, The inverse agonist propranolol confers no corticosteroid-sparing activity in mild-to- moderate persistent asthma, Clin. Sci. (Lond.) 127 (11) (2014) 635–643, https:// doi.org/10.1042/CS20140249.
41. P.M. Short, W.J. Anderson, P.A. Williamson, B.J. Lipworth, Effects of intravenous and oral β-blockade in persistent asthmatics controlled on inhaled corticosteroids, Heart 100 (3) (2014) 219–223, https://doi.org/10.1136/heartjnl-2013-304769.
42. D.A. Richards, B.N. Prichard, Clinical pharmacology of labetalol, Br. J. Clin. Pharmacol. 8 (Suppl 2) (1979) 89S–93S.
43. Pharmya, Questions and answers on FDA’s adverse event reporting system (FAERS), Available at: https://www.pharmya.com/wp-content/uploads /2023/11/USA-Questions-and-Answers-on-FDAs-Adverse-Event-Reporting-Syste m-FAERS-_-FDA_08Nov2023.pdf. (Accessed 21 October 2024).
44. T. Sakaeda, A. Tamon, K. Kadoyama, Y. Okuno, Data mining of the public version of the FDA adverse event reporting system, Int. J. Med. Sci. 10 (7) (2013) 796–803, https://doi.org/10.7150/ijms.6048.
45. S. Weiss-Smith, G. Deshpande, S. Chung, V. Gogolak, The FDA drug safety surveillance program: adverse event reporting trends, Arch. Intern. Med. 171 (6) (2011) 591–593, https://doi.org/10.1001/archinternmed.2011.89.
46. M.A. McAdams, L.A. Governale, L. Swartz, T.A. Hammad, G.J. Dal Pan, Identifying patterns of adverse event reporting for four members of the angiotensin II receptor blockers class of drugs: revisiting the Weber effect, Pharmacoepidemiol. Drug Saf. 17 (9) (2008) 882–889, https://doi.org/10.1002/pds.1633.
47. A.M. Hochberg, M. Hauben, R.K. Pearson, D.J. O’Hara, S.J. Reisinger, Systematic investigation of time windows for adverse event data mining for recently approved drugs, J. Clin. Pharmacol. 49 (6) (2009) 626–633, https://doi.org/10.1177/ 0091270009333484.
48. A. Pariente, F. Gregoire, A. Fourrier-Reglat, F. Haramburu, N. Moore, Impact of safety alerts on measures of disproportionality in spontaneous reporting databases: the notoriety bias, Drug Saf. 30 (10) (2007) 891–898, https://doi.org/10.2165/ 00002018-200730100-00007.
49. H.W. Wang, A.M. Hochberg, R.K. Pearson, M. Hauben, An experimental investigation of masking in the US FDA adverse event reporting system database, Drug Saf. 33 (12) (2010) 1117–1133, https://doi.org/10.2165/11584390- 000000000-00000.
50. E. Raschi, C. Piccinni, E. Poluzzi, G. Marchesini, F. De Ponti, The association of pancreatitis with antidiabetic drug use: gaining insight through the FDA pharmacovigilance database, Acta Diabetol. 50 (4) (2013) 569–577, https://doi. org/10.1007/s00592-011-0340-7.
51. Global Initiative for Asthma, Global strategy for asthma management and prevention (2024 update), Available at: https://ginasthma.org/wp-content/uploa ds/2024/05/GINA-2024-Strategy-Report-24_05_22_WMS.pdf. (Accessed 23 October 2024).
52. A. Niimi, K. Fukunaga, M. Taniguchi, et al., Executive summary: Japanese guidelines for adult asthma (JGL) 2021, Allergol. Int. 72 (2) (2023) 207–226, https://doi.org/10.1016/j.alit.2023.02.006.
53. Scottish Intercollegiate Guidelines Network, British Thoracic Society, SIGN158. British guideline on the management of asthma, Revised edition published July 2019. Available at: https://www.sign.ac.uk/media/1773/sign158-updated.pdf. (Accessed 23 October 2024).
54. National Asthma Education and Prevention Program, Expert panel report 3: guidelines for the diagnosis and management of asthma. Full report 2007, Available at: https://www.epa.gov/sites/default/files/2014-09/documents/asth gdln.pdf. (Accessed 23 October 2024).
55. R.B. Singh, J. Fedacko, A. Moshiri, et al., Chapter 28 - beta-blocker therapy among patients with heart failure, in: R.B. Singh, J. Fedacko, K. Hristova, G.N. Elkilany (Eds.), Pathophysiology, Risk Factors, and Management of Chronic Heart Failure, Academic Press, 2024, pp. 351–359, https://doi.org/10.1016/B978-0-12-822972- 9.00033-X.
56. M. Cazzola, C.P. Page, N.A. Hanania, L. Calzetta, M.G. Matera, P. Rogliani, Asthma and cardiovascular diseases: navigating mutual pharmacological interferences, Drugs (2024), https://doi.org/10.1007/s40265-024-02086-5. Online ahead of print.
57. Muresan, G. Cismaru, C. Muresan, et al., Beta-blockers for the treatment of arrhythmias: bisoprolol - a systematic review, Ann. Pharm. Fr. 80 (5) (2022) 617–634, https://doi.org/10.1016/j.pharma.2022.01.007.
58. N.Y. Grigorieva, T.P. Ilushina, K.S. Kolosova, The possibilities of using beta-blocker bisoprolol in patients with stable angina with concomitant bronchial asthma, Kardiologiia 62 (1) (2022) 32–39, https://doi.org/10.18087/cardio.2022.1.n1714.
59. Morales, D., Jackson, C., Lipworth, B., Donnan, P., & Guthrie, B. (2014). Adverse respiratory effect of acute β-blocker exposure in asthma: a systematic review and meta-analysis of randomized controlled trials.. Chest, 145 4, 779-786 . https://doi.org/10.1378/chest.13-1235.
60. Bennett, M., Chang, C., Tatley, M., Savage, R., & Hancox, R. (2021). The safety of cardioselective β1-blockers in asthma: literature review and search of global pharmacovigilance safety reports. ERJ Open Research, 7. https://doi.org/10.1183/23120541.00801-2020.
61. Huang KY, Tseng PT, Wu YC, Tu YK, Stubbs B, Su KP, Matsuoka YJ, Hsu CW, Lin CH, Chen YW, Lin PY. Do beta-adrenergic blocking agents increase asthma exacerbation? A network meta-analysis of randomized controlled trials. Sci Rep. 2021 Jan 11;11(1):452. doi: 10.1038/s41598-020-79837-3. PMID: 33432057; PMCID: PMC7801657